Current as of May 3, 2024
IRB Study Results
IRB Study Protocol: PR 24-006
PN 3047, Rev A
Study Design
The study employs a prospective observational design and can be conducted at up to 8 investigative sites. Up to 500 participants aged 18 or older, already scheduled for neurotoxin injections, can be enrolled. Additionally, participants must have received at least one previous neurotoxin injection to be enrolled in the study. After providing informed consent, participants receive injections using the NavaClick Injection System, following which both investigators and participants complete questionnaires assessing their experiences.
Touch-Up Data
Site 1 reports a significant reduction in touch-ups following the use of the NavaClick™ Injection System. A 'touch-up' is defined as “a patient returning with undesired clinical results from a neurotoxin procedure — within four weeks of injection — despite having received appropriate clinical dosing.” Of the 122 participants enrolled at Site 1, only one participant required a touch-up (a touch-up rate of 0.82%) compared with Site 1's 2023 touch up rate of 4.5%.
Overall Satisfaction
100% of investigators strongly agree they provide a higher standard of care with NavaClick™.
87% of participants reported a better overall experience.
82% of participants are likely to request it for future injections.
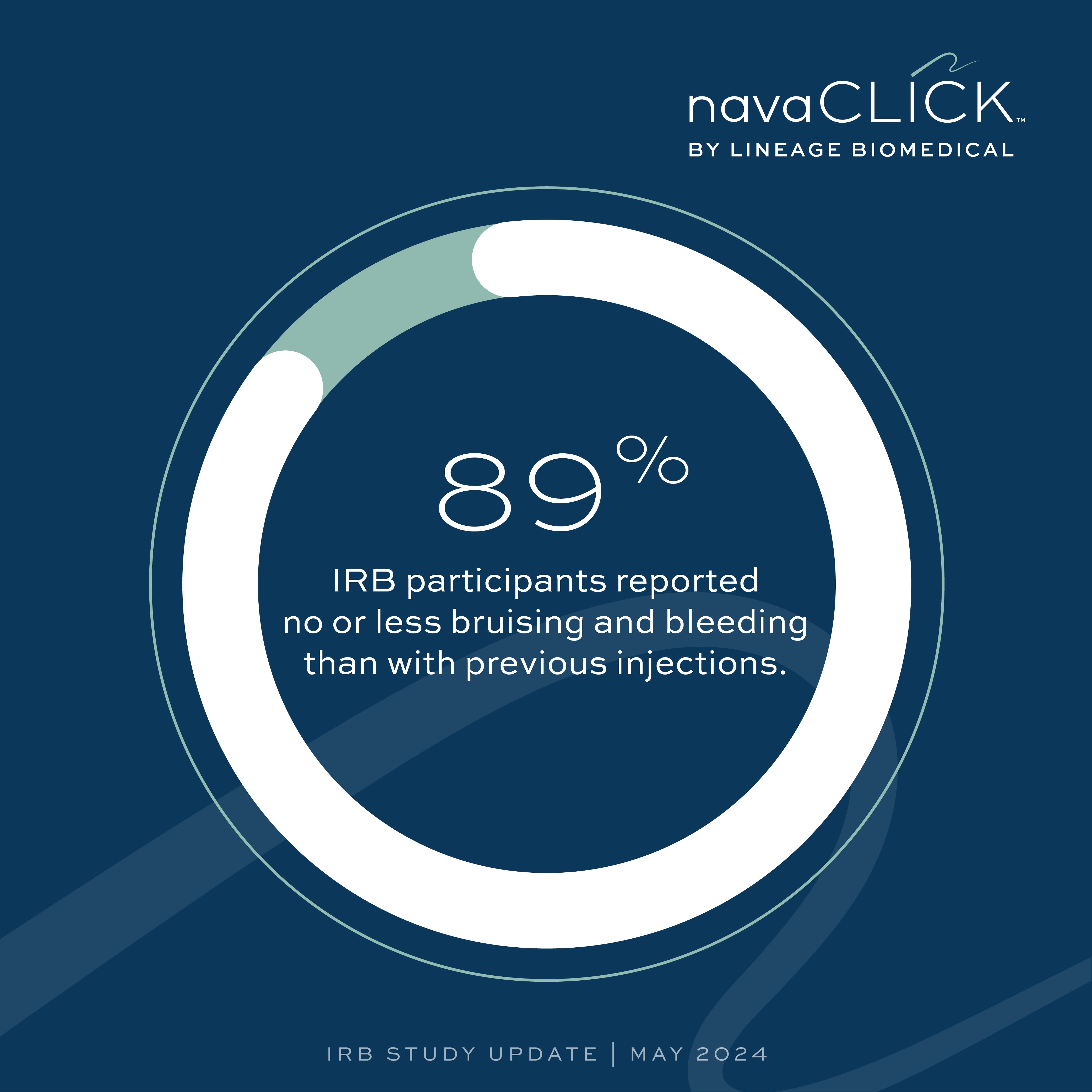
Fewer Complications
89% of participants reported experiencing either no bruising, bleeding, or inflammation or less than with past injections.
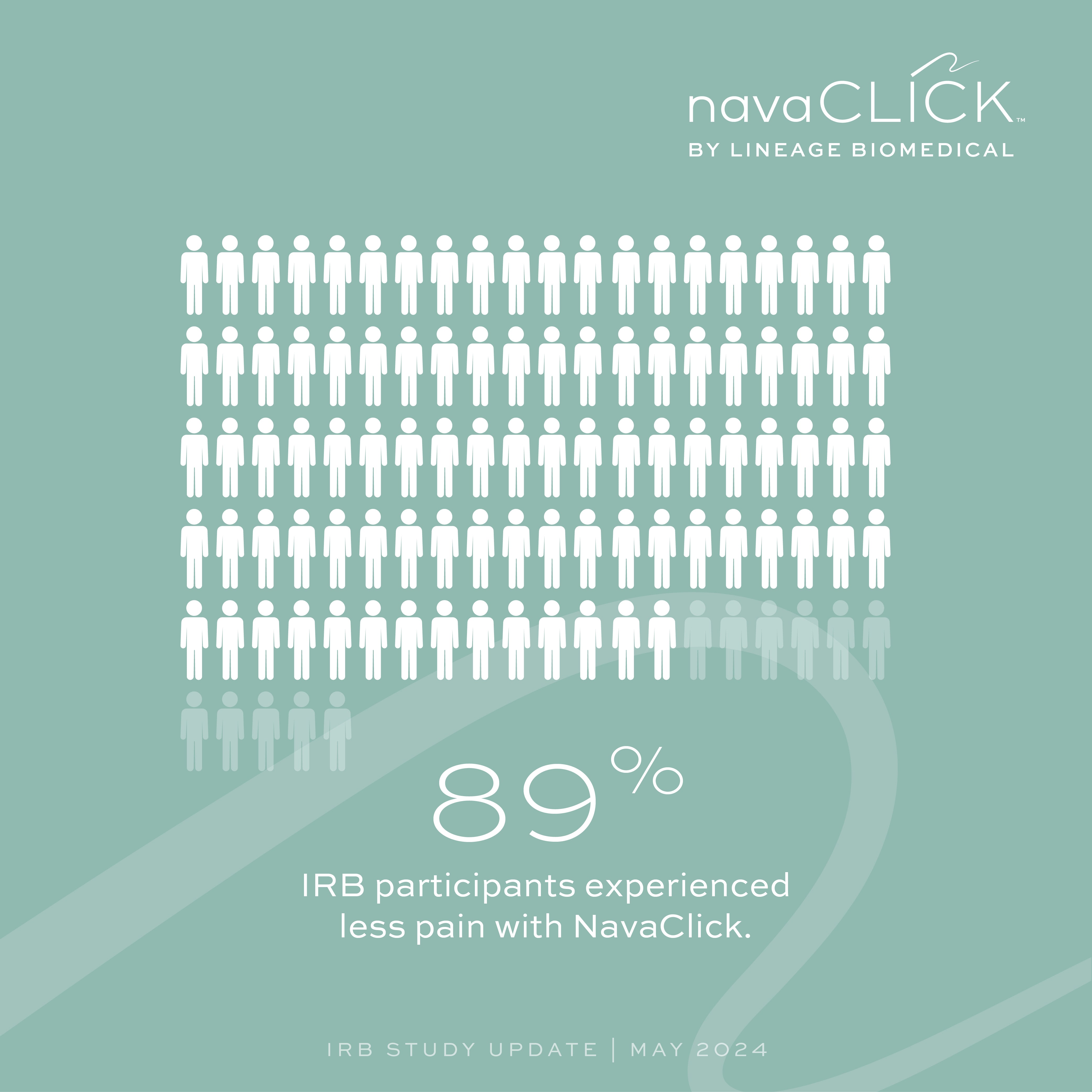
Less Pain
89% of participants experienced less pain with NavaClick™.
Simple. Accurate. Efficient.
100% of investigators strongly agreed that NavaClick simplifies the preparation and delivery of neurotoxins, provides greater site and dosing accuracy, improves overall efficiency, and reduces neurotoxin waste.
Click here to download copy of the IRB Study Results.